The United States healthcare system spends almost 10 billion dollars yearly treating the top HAIs (Hospital Associated Infections), one-third of which are from surgical site infections. Disinfection, handwashing, and sterilization prevent illnesses in a hospital setting.
Sterile processing, the procedure by which medical and surgical instruments are sterilized and cleaned for reuse, is one of the essential components of decreasing the perpetuation of HAIs. However, it is a routine maintenance that does not get the attention that it deserves. Without reasonable sterile processing, staff, patients, or anyone coming into contact with used medical instruments would be in danger of contracting a disease.
Sterile processing starts when non-disposable instruments utilized in surgical and medical procedures are transported to a decontamination area and cleaned in numerous ways to prepare for reuse. Tools are first taken to the decontamination area, then disassembled and cleaned with detergent or enzymatic solution before moving in an ultrasonic cleaner, keeping it in the washer, and packing for sterilization.
Hospitals have various ways of conducting sterile processing programs, but the CDC has guidelines to ensure that certain norms are met regardless of variations in hospital staff. Six main stages of sterile processing should be managed at a high level. They are:
For each stage, the CDC comprises specific directions regarding which cleaning products and tools can be used, how to specify when successful sterilization happened, and what kinds of assessments should take place before instruments are moved to the next stage.

Sterile processing technicians must be diligent in conserving these norms, so they themselves stay safe and that neither medical staff nor patients are exposed to hazardous pathogens. These technicians are the unsung heroes of the hospital because they have an important job but are not recognized for their role in improving outcomes and decreasing risks.
Beginning in 2015, the FDA began a comprehensive study and follow-up procedure to reinforce sterile processing guidelines for duodenoscopes. These are the devices used in some gastroenterology methods. Because duodenoscopes have small and complex parts, they are hard to clean. The FDA in 2019 found the following as a result of their study:
The FDA proceeds to monitor the sterile processing job of duodenoscopes to decrease patient risk and the incidence of infection. As this study indicates, following sterile processing guidelines has a crucial impact on patient safety.
There are advances in the production of surgical tools that also provide a greater extent of patient safety. Manufacturers are now developing devices that are easier for the sterile processor to clean and sustainably-produced single-use components that offer high performance and are an eco-friendly option to traditional plastic disposables.
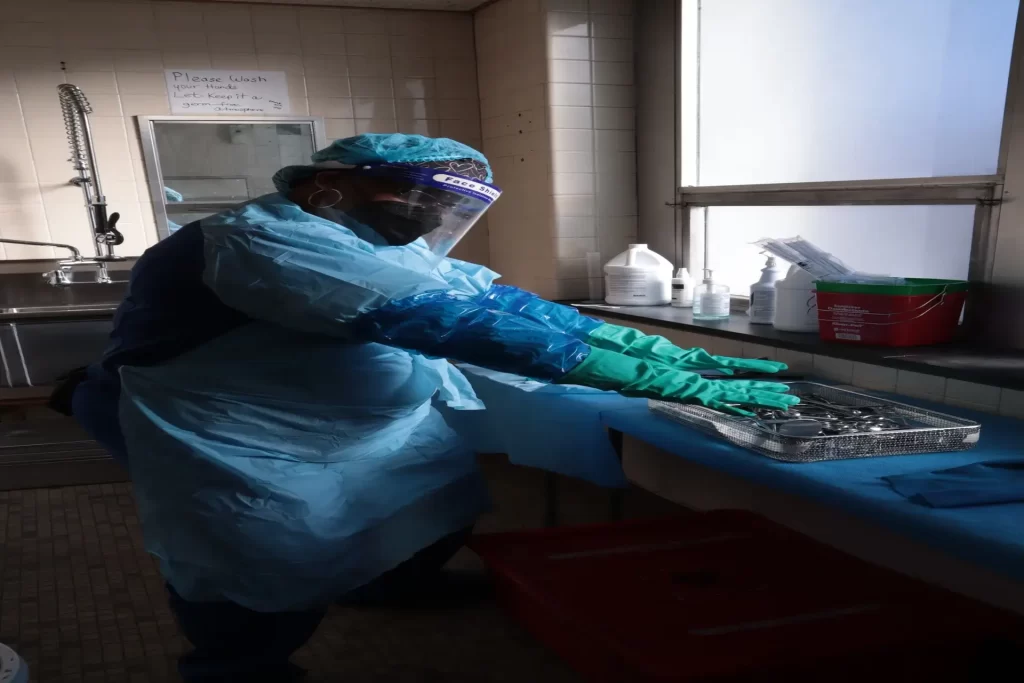
Sterile processing proceeds to be a crucial part of quality patient care. As this relatively unknown field goes on to advance, opportunities for improved outcomes and patient safety will only rise.
Sterilization methods should be monitored using biological, mechanical, and chemical indicators. The biological indicators are the most accepted means of monitoring sterilization by sterile techs because they assess the sterilization procedure directly by killing known highly resistant microorganisms. Nonetheless, chemical and mechanical monitoring should also be done because spore tests are only done weekly, and the outcomes are usually not obtained immediately.
Mechanical and chemical indicators don’t guarantee sterilization; they help detect procedural mistakes and equipment malfunctions. Therefore, chemical and mechanical monitoring should be done for every sterilizer load.
The records of sterilization monitoring (biological, mechanical, and chemical) should be retained long enough to comply with state and local regulations. Unfortunately, the CDC (Centers for Disease Control and Prevention) does not maintain data on time limits for every state but provides an example of three years in its sterilization guidelines, which is the time frame utilized by the Joint Commission inspection agency.
Read More